A) alpha particle.
B) proton.
C) beta particle.
D) neutron.
E) gamma ray.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The most widely used medical isotope in nuclear medicine is
A) I-131.
B) I-125.
C) P-32.
D) Co-60.
E) Tc-99m.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Essay
The radiation dose required to produce death in one-half of the exposed subject animals is termed the ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is missing in the nuclear reaction shown below? 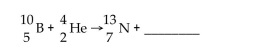
A) a neutron
B) gamma radiation
C) an alpha particle
D) a beta particle
E) a positron
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What particle is emitted in the following nuclear reaction?
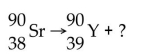
A) beta particle
B) gamma ray
C) neutron
D) proton
E) alpha particle
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A wooden object from a prehistoric site has a carbon-14 activity of 10 counts per minute (cpm) compared to 40 cpm for new wood. If carbon-14 has a half-life of 5730 years, what is the age of the wood?
A) 22,900 yr
B) 1430 yr
C) 17,200 yr
D) 5730 yr
E) 11,500 yr
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Essay
The diagnostic imaging technique that depends on magnetic fields and radio waves, not radioactivity, is called ________.
Correct Answer

verified
MRI or mag...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
One symptom of mild radiation sickness is
A) a raised white cell count.
B) a raised red blood cell count.
C) a lowered white cell count.
D) a lowered red blood cell count.
E) a white cell count of zero.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A person begins to suffer radiation sickness at an exposure level of
A) 600 rem.
B) 25 rem.
C) 100 rem.
D) 5 rem.
E) 500 rem.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is NOT a way to minimize your exposure to radiation?
A) staying a longer time
B) wearing a lead apron
C) keeping a good distance
D) wearing lead-lined gloves
E) standing behind a thick concrete wall
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is suitable as a minimum shielding for beta particles?
A) 1 m of water
B) 1 m of concrete
C) gloves
D) air
E) 1 cm of lead
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Gamma rays require the heaviest shielding of all the common types of nuclear radiation because gamma rays have the
A) lowest energy.
B) most intense color.
C) largest particles.
D) heaviest particles.
E) highest energy.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is it important that radioisotopes used in diagnostic tests have short half-lives?
A) These radioisotopes are less expensive.
B) These radioisotopes are more abundant in nature.
C) These radioisotopes have a greater activity so they are easier to monitor.
D) This is necessary so the radioisotopes will have high energy.
E) This minimizes the harmful side effects of the radiation.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
The time needed for a radioactive sample to decay to one-half of its original activity is called the ________.
Correct Answer

verified
Correct Answer
verified
Essay
Write the word or phrase that best completes each statement or answers the question.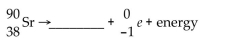
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of cerium-141 for a diagnostic test was dissolved in saline solution to an activity of 4.5 mCi/mL. If the patient undergoing the test needs a dose of 10. mCi, how much of the solution should be injected into the Patient?
A) .45 mL
B) 2.2 mL
C) 4.5 mL
D) 22 mL
E) 45 mL
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Sodium-24 has a half-life of 15 hours. How many hours is three half-lives?
A) 15 hours
B) 45 hours
C) 7.5 hours
D) 30 hours
E) 60 hours
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A patient receives 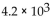 mrads of iodine-131, which emits
mrads of iodine-131, which emits 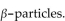 If the factor that adjusts for biological damage is 1 for beta particles, how many rems did the patient receive?
If the factor that adjusts for biological damage is 1 for beta particles, how many rems did the patient receive?
A) 2.0
B) 4.0
C) 40
D) 0.40
E) 0.30
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Radium-226 decays by alpha decay to
A) barium-131.
B) cobalt-60.
C) radon-222.
D) polonium-218.
E) carbon-14.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If absorbed internally, alpha particle emitters are the most damaging because alpha particles
A) have the greatest mass.
B) consist of high energy electrons.
C) have the greatest energy.
D) consist of pure energy.
E) have the largest charge.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 64
Related Exams